6-溴-3-(1-甲基-1H-吡唑-4-基)-5-(3R)-3-哌啶基吡唑并
發(fā)布者:信康 瀏覽次數(shù):
產(chǎn)品名稱:6-溴-3-(1-甲基-1H-吡唑-4-基)-5-(3R)-3-哌啶基吡唑并[1,5-A]嘧啶-7-胺CAS:891494-63-6別名:6-溴-3-(1-甲基-1H-吡唑-4-基)-5-(3R)-3-哌啶基吡唑并[1,5-A]嘧啶-7-胺;SCH900776(R型);SCH900776(S型)英文名:SCH900776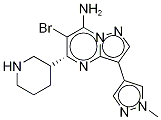 []
[] - 生物活性MK-8776 (SCH 900776)是一種選擇性Chk1抑制劑,IC50為3 nM��,比作用于Chk2選擇性高50倍。Phase 2�。
- 體外研究SCH 900776 is not a potent inhibitor of Chk2 and CDK2 with IC50 of 1.5 μM and 0.16 μM, respectively. SCH 900776 shows no significant inhibition of cytochrome P450 human liver microsomal isoforms 1A2, 2C9, 2C19, 2D6, and 3A4. SCH 900776 induces a dose-dependent loss of DNA replication capability 24 hours after hydroxyurea exposure. SCH 900776 enhances the γ-H2AX response of hydroxyurea, 5-fluoruracil, and cytarabine. In combination with an antimetabolite, SCH 900776 induces accumulation of γ-H2AX within 2 hours, indicative of replication fork collapse and double stranded DNA breaks. Additionally, SCH 900776 suppresses accumulation of the Chk1 pS296 autophosphorylation in a dose-dependent manner. Exposure of proliferating WS1 cells to SCH 900776 is associated with rapid, dose-dependent accumulation of Chk1 pS345, indicating that cycling populations of normal cells induce Chk1 pS345 following exposure to SCH 900776 as part of a futile cycle, perhaps driven by AT-family kinases and DNA-PK.
- 體內(nèi)研究Administered 30 minutes after gemcitabine, 4 mg/kg SCH 900776 is sufficient to induce the γ-H2AX biomarker while 8 mg/kg leads to enhanced tumor pharmacodynamic and regression responses relative to gemcitabine or SCH 900776 alone. Dose escalation of SCH 900776 (16 mg/kg and 32 mg/kg) induces incremental improvements in tumor response. Importantly, doses of SCH 900776 associate with robust biomarker activation and improved tumor response are not associated with enhanced toxicity of gemcitabine on hematological parameters in BALB/c mice.
 []
[]